Exclusive Representations
IBS Angel™ – Absorbable Scaffold
IBS Angel™ – Innovation in pediatric interventional cardiology

IBS Angel™ IBS Angel™ is the first bioabsorbable iron scaffold developed to accompany the growth of neonatal patients requiring vascular treatment. Designed especially for congenital heart diseases (ductus dependent), it offers an innovative solution that combines effectiveness, safety and adaptability.
Unlike permanent metal stents, IBS Angel™ provides temporary support and then degrades naturally. It maintains its mechanical strength for the first few months, begins to reabsorb from the third month and disappears completely in a period of 2 to 3 years. In this way, it allows the glass to recover its normal function and avoids the rigidity generated by traditional devices.
Its composition is based on a pure nitrid iron core, with zinc coatings and polylactic acid polymer, and gold radiopaque markers that facilitate its visualization during the procedure.
This advance offers key benefits for specialists and their patients:
- It accompanies the natural growth of the child without leaving permanent implants.
- It facilitates future interventions in the same area without damaging the tissues.
- Biocompatible and safe, with clinical studies that support its effectiveness.
- Internationally validated innovation, with CE MDR certification and FDA approval in cases of “Compassionate Use”.
Product Design
Backbone
Made from a nitrided iron tube with excellent mechanical properties(radial strength), which can provide effective support for blood vessels.
Re-intervention
Compared with permanent stents, the lBS Angel™ scaffold is degradable, and re-intervention is feasible in the same position without causing harm or adding burden to the blood vessels or the human body.
Degradation
The degradation period is about 2-3 years. lt provides effective support for as the infant growth. The scaffold starts to degrade around 3 months after implantation when the blood vessel recovers. The scaffold will be absorbed by the body 2 years after implantation.
Indication
The indication of the lBS Angel™ lron Bioresorbable Scaffold is to improve the lumen diameter of the ductus arteriosus of newborns and infants who require stent treatment, with reference vessel diameters of 2.25 mm to 4.25 mm and target segment length ≤ 35 mm.
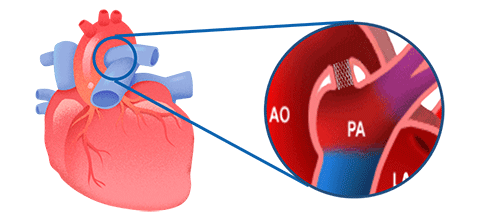
IBS Angel™ represents a paradigm shift in the treatment of congenital heart disease: a technology designed to offer support today and freedom for the future.
Siprotec S.A.
Comprehensive Prostheses
Delivery System.
Address
Buenos Aires, Argentina
Tel./Whatsapp: 11 2746 0165
Contacts
Suppliers: proveedores@siprotec.com.ar
Collections: cobranzas@siprotec.com.ar
Quotes: cotizaciones@siprotec.com.ar