Six-Month Outcomes of the TricValve® System in Patients with Tricuspid Regurgitation: TRICUS EURO Study
ABSTRACT
Background
Severe tricuspid regurgitation (TR) is frequently associated with significant morbidity and mortality; such patients often deemed to be at high surgical risk. Heterotopic bi-caval stenting is an emerging, attractive transcatheter solution for these patients.
Objectives
To evaluate the 30-day safety and 6-month efficacy outcomes of specifically designed bioprosthetic valves for the superior and inferior vena cava.
Methods
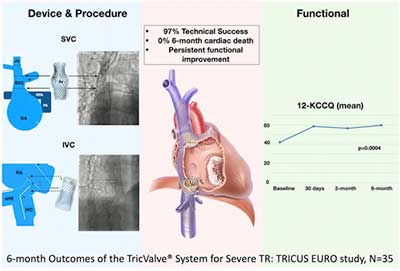
TRICUS EURO is a non-blinded, non-randomized, single-arm, multicenter, prospective trial that enrolled patients from 12 European centers between December 2019 to February 2021. High risk individuals with severe symptomatic TR despite optimal medical therapy were included. Primary end point was quality of life (QOL) improvement measured by Kansas City Cardiomyopathy Questionnaire (KCCQ12) and New York Heart Association functional class (NYHA) improvement at 6-month follow-up.
Results
35 patients (mean age 76±6.8 years; 83% women) were treated with TricValve® system. All patients at baseline were at NYHA ≥ 3 status. At 30-days, procedural success was 94% with no procedural deaths or conversions to surgery. A significant increase in QOL at 6-months follow-up was observed (baseline and 6-month KCCQ: 42.01±22.3 vs. 59.7±23.6 respectively; p=0.004), correlating with a significant improvement in NYHA functional class with 79.4% of patients noted to be in class I or II at 6 months (p=0.0006). The 6-month all-cause mortality and heart failure hospitalization rates were 8.5% and 20%, respectively.
Conclusions
The dedicated bi-caval system for treating severe, symptomatic TR was associated with high procedural success rate and significant increase in both, QOL and functional improvements at 6-months follow-up.